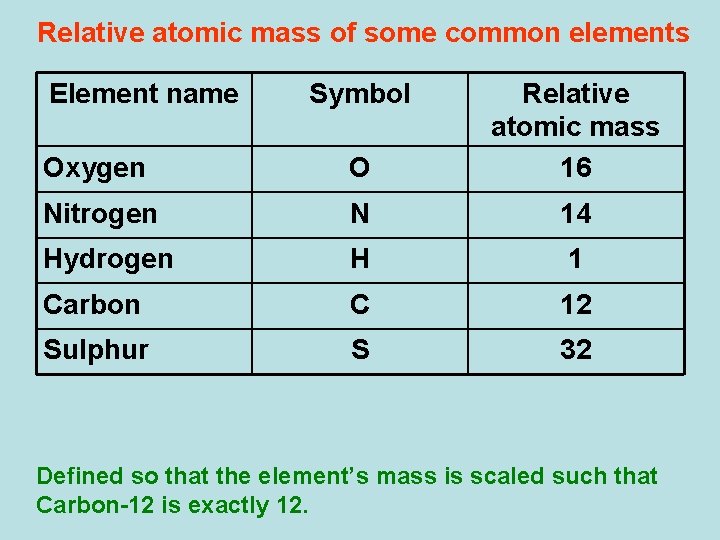
The unit for the relative atomic mass is dalton (Da) or also called the Unified Atomic Mass unit (u). It is 1/12th of the mass of a carbon-12 isotope. Note: this definition will change when IUPAC. Oxygen: O: 15.9994: 1: 20.114%. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation. The relative atomic mass is the average atomic mass relative to the atomic mass Carbon-12. 'different relative atomic mass' therefore means in fact that Mars and Earth oxygen have different mixes of oxygen isotopes. Chemical processes that occur on planets and in space can partially separate, or fractionate, the different isotopes. Given that the relative atomic mass (Ar ) of oxygen is 16, what is the metal in the metal oxide? The formula of the metal oxide is MO, which means there is only one oxygen atom bonded to the metal. The Mr of a compound is the sum of Ar of the elements that make up the compound.
Related Topics: More Chemistry LessonsIn this lesson, we will learn
- how to calculate the relative formula mass or relative molecular mass
- how to use the relative molecular mass to calculate the percent mass of an element in a compound
- how to use the relative molecular mass to calculate the percent mass of water in a compound
Relative Atomic Mass How to Find Relative Atomic Mass of an Element? Define Relative Atomic Mass - YouTube. If playback doesn't begin shortly, try restarting your device. Videos you watch may be.
The following diagram shows how to calculate the relative molecular mass or relative formula mass. Scroll down the page for more examples and solutions.
What is relative formula mass and relative molecular mass?
The relative formula mass of a substance is the sum of the relative atomic masses of the elements present in a formula unit. The symbol for relative formula mass is Mr.
If the substance is made of simple molecules, this mass may also be called the relative molecular mass.
Example:
What is the relative mass formula of hydrogen gas? (Relative atomic mass: H = 1)
Solution:
The formula for hydrogen gas is H2. Each molecule contains 2 hydrogen atoms.The relative mass formula of hydrogen gas is
Mr(H2) = 2 × Ar(H) = 2 × 1 = 2
Example:
What is the relative mass formula of water? (Relative atomic masses: H = 1, O = 16)
Solution:
 The formula for water is H2O. Each molecule contains 2 hydrogen atoms and 1 oxygen atom.
The formula for water is H2O. Each molecule contains 2 hydrogen atoms and 1 oxygen atom.The relative mass formula of water is
Mr(H2O) = 2 × Ar(H) + Ar(O) = 2 × 1 + 16 = 18
Example:
What is the relative mass formula of sodium chloride? (Relative atomic masses: Na = 23, Cl = 35.5)
Solution:
Sodium Chloride is an ionic solid with the formula Na+Cl-.The relative mass formula of sodium chloride is
Mr(NaCl) = Ar(Na) + Ar(Cl) = 23 + 35.5 = 58.5
How to find the Percent Mass of Elements in a compound?
How to use Mr to calculate the percent mass of an element in a compound?
Example:
What percentage of the mass of ammonium nitrate is nitrogen? (The formula for ammonium nitrate is NH4NO3, Relative atomic masses: H = 1, O = 16, N = 14)
Solution:
Mr(NH4NOThe Relative Atomic Mass Of Oxygen Is 16 Explain Its Meaning
3) = (2 × 14) + (4 × 1) + (3 × 16) = 28 + 4 + 48 = 80Mass of nitrogen in the formula = 28
Mass of nitrogen as a fraction of the total =
Relative Atomic Mass Example
Mass of nitrogen as percentage of total mass
Example:
What percentage of the mass of O in NaNO3? (Relative atomic masses: Na = 23, O = 16, N = 14)
Solution:
Mr(NaNO3) = 23 + 14 + (3 × 16) = 23 + 14 + 48 = 85
Mass of O in the formula = 48Mass of O as a fraction of the total =
Mass of O as percentage of total mass
How to find percent by mass and percent composition?
Example:
Find the percent composition by mass of potassium dichromate (K2Cr2O7)
Step 1: Find the molar mass of the compound.
Step 2: Divide the total mass of each element by the molar mass and multiply by 100 to find the % mass.
- Show Step-by-step Solutions
Finding the percent by mass means finding the mass of the elements in the compound and adding the masses for the total mass.
Example:
You have a 7364 milligrams sample of SO2. How many grams of sulfur are in the sample?
How to calculate the Mass Percent of an Element in a Compound?
Mass Percent Composition of an Element in a Compound
To calculate the mass percent composition (or simply, the mass percent) of an element in a compound, we divide the mass of the element in 1 mol of the compound with the mass of 1 mol of the compound and multiply by 100%.
Examples:
1. What is the mass percent of carbon in carbon dioxide?
2. What is the mass percent of oxygen in carbon dioxide?
- Show Step-by-step Solutions
How to use Mr to calculate the percent mass of water in a compound?
Example:
What percentage of the mass of magnesium sulfate is water? (Given that the formula for magnesium sulfate is MgSO4•7H2O, Relative atomic masses: H = 1, O = 16, S = 32, Mg = 24)
Solution:
Mr(MgSO4•7H2O) = 24 + 32 + (4 × 16) + (7 × 18) = 246Mr(H2O) = 2 × Ar(H) + Ar(O) = 2 × 1 + 16 = 18
Mass of water in the formula = 18 × 7 = 126
Mass of water as a fraction of the total =
Mass of hydrogen as percentage of total mass
How to calculate the Water of Crystallization?
This video outlines how to determine the percent, by mass, of water trapped in a hydrate's crystal lattice.
Example:
What is the percent of water, by mass, in the hydrate CuSO4•2H
 2O How to calculate the percentage of water in the formula of a hydrated compound?
2O How to calculate the percentage of water in the formula of a hydrated compound?Example:
Determine the % water in the following hydrates:
a) CuSO4•5H2O
b) CaCl2•2H2O
c) KAl(SO4)2•12H2O
- Show Step-by-step Solutions
Try the free Mathway calculator and problem solver below to practice various math topics. Try the given examples, or type in your own problem and check your answer with the step-by-step explanations.
We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.
Helo, reders welcome to “textilesgreen.in” today i m going to explain about molar mass of h2o.
i have made this guide to help you out.
So, hold your seat and be with the end of guide.
So you could get valuable information out of it.
lets get started;
Read More:- Molar mass of CO2?
What is molar mass?
It is define as the mass of sample of that chemical compound divided by the amount of substance in that sample. this phenomenon is called molar mass.
It is measured in moles.
the molar mass of element it can represent gram atomic mass.
the molar mass of compound it can represent gram molecular mass.
If you want to find the Molar mass of chemical compound, such as h2o then you can follow this process.
the relative atomicmass of an element expressed in gram is called gram atomic mass.
For example;
Gram atomic mass of oxygen is = O = 16g
The relative atomic mass of an compound expressed in grams is called gram molecular mass.
For example;
O+O = 16+16 = 32 g
Where, O = oxygen
H+O+H = 1+16+1= 18g
Where, H = Hydrogen and O= oxygen
Read More:- Molar Mass of NaCl?
You can understand easily with the help of following structure.
You know,
1 unit of hydrogen = one atoms of hydrogen.
1 gram of hydrogen = one mole of hydrogen.
Where,
Value of 1 gram of hydrogen is = 6.022×10 rase power of 23.
Similarly,
About H2O (water)
18 units = one molecule of water
18 gram = one moles of water.
You know that units is much smaller then grams.
Find the molecular mass of h2o (h2O molar mass)
Find the molecular mass of h2o with the following basic information.
Such as,
First of all, You know that,
atomic mass of hydrogen = 1
atomic mass of oxygen = 16
Where, H= Hydrogen or O = oxygen.
What Is Relative Atomic Mass
In this chemical compound, it has two atom of Hydrogen and one atom of oxygen is present.
Relative Atomic Mass List
So, two Hydrogen = H2= 1+1 = 2.
Where, atomic mass of hydrogen= 1
But here, two Hydrogen is persent in h2o.
Relative Atomic Mass Of Oxygen
So, 1+1 = 2 or multiple by 2, in both cases, it will same meaning. and one oxygen is persent in h20.

it mean, O= oxygen, atomic mass of O = 16
Where,
Atomic mass of oxygen is 16
So, For determine the molar mass of h20 add both atomic mass.
Relative Formula Mass Of Oxygen
Now, atomic mass of h20 = 1+1+16 = 18 gram
So, molar mass of h2o is = 18 gram.
The molar mass of chemical compound is represente by gram molecular mass.

So, it is represente by “Gram”
the final result is,
Molar Mass of h2o is 18 gram.
Also read;
• Full form of h2o
• Nacl full form
